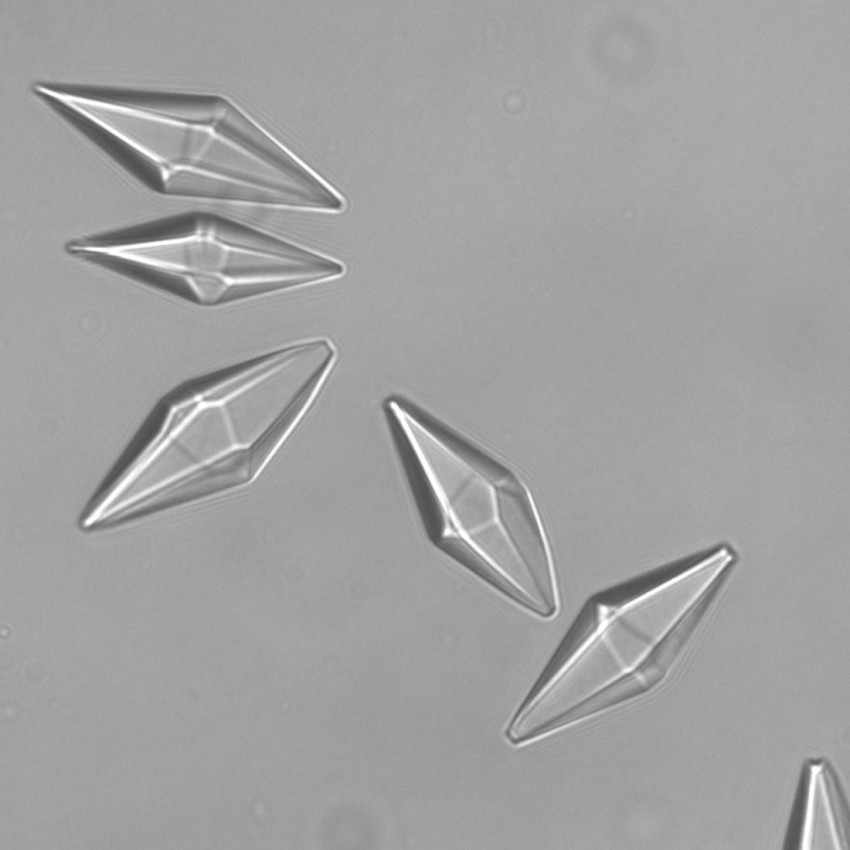
Mineral binds CO2 during hydrogen production
Producing hydrogen from natural gas releases CO2. However, this greenhouse gas is easy to bind using a solid substance, so you can store it underground at a later date or use it as a raw material. This has been demonstrated by the research of Kai Coenen at Eindhoven University of Technology, for which he received his doctorate last Tuesday, 9 October.
Hydrogen is still commonly produced from natural gas. Together with steam, the gas (methane) enters a so-called reformer, where it is converted into syngas, a mixture of carbon monoxide and hydrogen (see the first reaction below). In the second reaction, the carbon monoxide reacts with water, resulting in carbon dioxide and – once again – hydrogen.
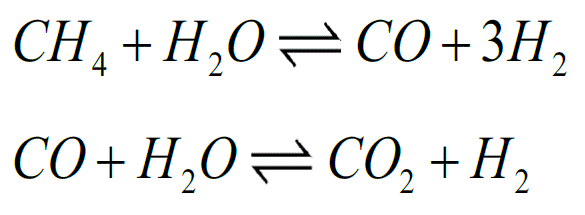
Because the second reaction is a balanced reaction, you can increase the yield of the right-hand part by removing products of the reaction. In more concrete terms: if you bind the CO2 to something, you produce more hydrogen. This is the focus of Kai Coenen's doctoral thesis at Eindhoven University of Technology.
MIXTURE OF METAL OXIDES
He studied solids that bind to CO2 molecules. You can then extract the greenhouse gas in a concentrated form from these substances. He primarily focused on so-called hydrotalcite, a mixture of metal oxides, such as aluminium oxide, magnesium oxide and potassium carbonate. ‘This substance occurs in nature, but we focused on the artificial form, as it allows us to adjust the properties optimally to bind CO2 as well as possible,’ explains Coenen in a call from Ludwigshafen, where he is now working for a German chemical giant.
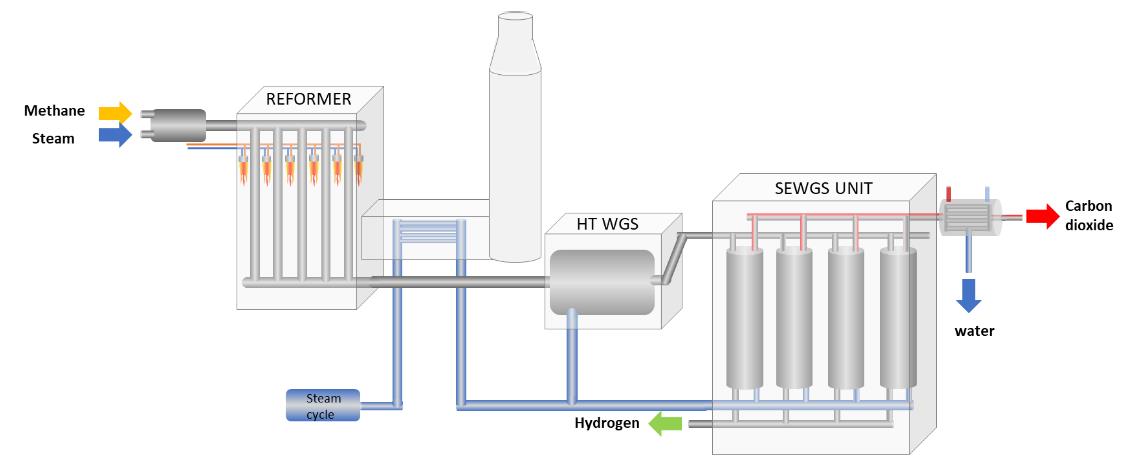
The idea is to place hydrotalcite in the form of compressed, porous granules with a cross-section of 4.5 mm (as in the figure above, the 'SEWGS unit') and to run the syngas (CO and H2) through it. The carbon dioxide binds to the granules, and the hydrogen flows through to storage tanks. Once the granules are saturated, the CO2 can be removed by passing steam through them.
SPEED CALCULATIONS
Coenen experimented with this process, discovering how fast it takes place. He also learnt a lot about the link between the shape of the surface of the hydrotalcite and binding CO2. Conclusion: extracting the CO2 is the most important step to make the entire process more efficient (from natural gas to hydrogen). ‘It was already known that steam 'pulls' CO2 more strongly than nitrogen, but my model enables you to calculate the speed.’
CONCENTRATED CO2 FLOW
The benefit of CO2-binding material is that a concentrated flow of CO2 remains. And that's convenient if you want to use it as a useful raw material for a subsequent process step. And if the government mandates the capture of CO2, then hydrotalcite is a good candidate.
Coenen's doctoral study is complete, but his model could serve as a springboard to a model that describes process flows. This would enable you to calculate, for instance, how many columns with hydrotalcite would be optimal in a factory. A trial factory is currently running in Sweden that uses hydrotalcite. Energieonderzoek Centrum Nederland (ECN, part of TNO) is also working on this project.
If you found this article interesting, subscribe for free to our weekly newsletter!
Opening photo: piece of hydrotalcite with a length of 8.4 cm, found in Norway. Source: iRocks.com / CC-BY-SA-3.0. Other images: Kai Coenen, PhD Thesis, 2018.